Applikon AppliFlex ST GMP - The single-use bioreactor for clinical applications
The AppliFlex ST GMP* is a customizable single-use bioreactor that is designed to meet all cGMP requirements for clinical CGT applications. It enables a seamless process transition from R&D to clinical production.
Single-use bioreactor for Cell & Gene Therapy and mRNA applications
- Seamless process transition from research to clinical production (both scale up and scale out)
- Designed to meet the requirements for clinical production, such as material requirements, traceability, particle requirements and much more
- Builds on the proven customization ability of the AppliFlex ST for research and development purposes

Turnkey package
Leveraging our expertise, we can provide a turnkey lab-scale cGMP manufacturing platform to get you started right away. This package consists of the single-use AppliFlex ST GMP bioreactor, a control tower, software solutions and services like installation, verification and training.
Features of the AppliFlex ST GMP Version
- Easy scale up and scale out:
Following the strictest cGMP standards, the AppliFlex ST GMP reduces the challenges of process transfer to clinical production. The AppliFlex ST GMP leverages your development and validation work into a GMP design and production workflow for 500 mL and 3 L bioreactor systems.
Save time and costs by skipping the process transfer step moving to clinical production, and thereby reduce your time-to-market.
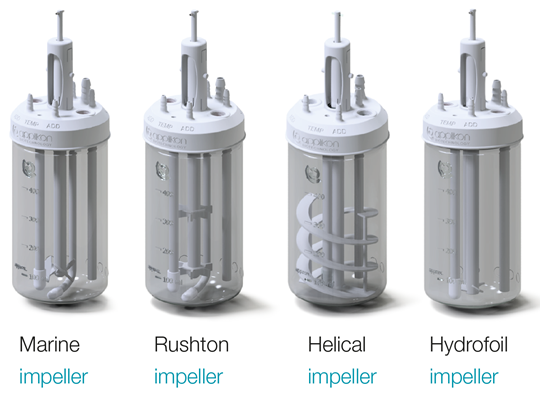
- Full customizable design
3D printing technology allows for easy optimization of the bioreactor to match your process and application. The headplate can be configured to your unique needs from special ports, sampling lines, connectors, and spargers to addition bottles.
The unique design and verification of the AppliFlex ST GMP allows you to keep this flexibility while being compliant with cGMP standards. Bring your design to life with a custom AppliFlex ST GMP.
- No cross-contamination
The AppliFlex ST GMP is a fully closed system. A wide range of aseptic connectors can be placed to reduce handling operations. Design has been optimized to reduce handling and make operation easy.
Using single-use sensors allows for a fully closed operation, ensuring sterility in your bioprocess.
Conclusion headline
With the Applikon AppliFlex ST GMP, you don’t have to make any compromises in the design of your bioprocess or the use for the dedicated purpose. You can make the risk of cross-contamination between runs a thing of the past! It also alleviates time-consuming cleaning and sterilizing since the system will be disposed of after use. And it enables a seamless process transition from R&D to clinical production