Applications
The mRNA Process: From Lab to Therapy
The process of developing messenger RNA (mRNA) for therapeutic applications is a sophisticated journey that merges the realms of molecular biology, genetic engineering, and bioprocess technology. It requires a series of precisely controlled steps, from the initial design of the mRNA sequence to its delivery into patient cells. This process harnesses the power of mRNA to instruct cells to produce specific proteins that can prevent, treat, or cure diseases. Below, we delve into the scientific intricacies of transforming a theoretical mRNA sequence into a viable therapeutic agent.
1. mRNA sequence design and optimization
The journey begins with the design of the mRNA sequence. This is not merely a transcription of the gene of interest but an optimized sequence that enhances the mRNA's stability, translational efficiency, and overall therapeutic efficacy. Scientists employ various strategies, such as codon optimization, to ensure that the host cell's machinery efficiently converts the mRNA into protein. Additionally, untranslated regions (UTRs) are engineered, and poly(A) tails are added to increase mRNA stability and lifespan in the cytoplasm.
2. In Vitro Transcription (IVT)
Once the mRNA sequence is designed and synthesized, the next step is in vitro transcription (IVT). This process involves using a DNA template—containing the T7, T3, or SP6 promoter—along with nucleotides, RNA polymerase, and other necessary components, to synthesize the mRNA molecule in a laboratory setting. The IVT reaction is carefully optimized to maximize the yield and purity of the mRNA product.
3. Purification and quality assurance
Following IVT, the mRNA is purified to remove any double-stranded RNA impurities, enzymes, and unincorporated nucleotides, as these can trigger immune responses or reduce the therapeutic efficacy. Purification techniques such as HPLC or affinity chromatography are employed. The purified mRNA undergoes rigorous quality control tests, including assessments of purity, concentration, integrity, and the presence of any contaminants.
4. mRNA encapsulation and delivery
Naked mRNA is inherently unstable and susceptible to degradation by ribonucleases present in bodily fluids. It is often encapsulated within lipid nanoparticles (LNPs) or other delivery vehicles to protect the mRNA and facilitate its entry into cells. This encapsulation process also involves optimizing the size, charge, and composition of the LNPs to ensure efficient cellular uptake and endosomal escape, allowing the mRNA to reach the cytoplasm where it can be translated into protein.
5. Scaling up production with bioreactors
Scaling up the production of mRNA for therapeutic use necessitates the use of bioreactors, such as the Applikon Mini bioreactor, which provide a controlled environment for the growth of the cells required for the IVT process. Bioreactors enable the precise control of conditions such as temperature, pH, and aeration, ensuring optimal cell density and health for high-yield mRNA production. This step is crucial for meeting the demands of clinical trials and commercial-scale manufacturing.
6. Clinical application: administration and monitoring
Depending on the target disease and tissue, the final mRNA therapeutic product is administered to patients through various routes. Following administration, patients are closely monitored for therapeutic outcomes and any adverse reactions. The efficacy of the therapy, measured by the expression level of the target protein and clinical improvement, along with safety profiles, are rigorously evaluated.
The development of mRNA therapeutics from lab to therapy encapsulates the complexity and potential of this cutting-edge field. Each step, from the initial design of the mRNA molecule to its delivery into patient cells, underscores the importance of precision, optimization, and control—hallmarks of the scientific endeavor to harness mRNA for therapeutic purposes.
Applications of mRNA Technology
Messenger RNA (mRNA) technology has rapidly advanced to the forefront of biomedicine, offering novel therapeutic applications across various diseases. This technology exploits the fundamental biological process of translating mRNA into proteins within cells. By designing synthetic mRNA that encodes for specific therapeutic proteins, scientists can prompt cells to produce these proteins in situ, providing a unique approach to treatment. Below, we explore the scientific intricacies and potential applications of mRNA technology in greater detail.
The role of the bioreactors in mRNA production
The Applikon Mini bioreactor stands out as a critical tool in the production of mRNA, offering several key advantages:
- Precision and control: It allows for precise control over the cultivation environment, which is crucial for producing high-yield mRNA.
- Scalability: The Applikon Mini bioreactor, designed to facilitate both research and commercial-scale production, supports the scalable manufacturing of mRNA therapeutics.
- Integration: It is compatible with various cell culture systems and bioprocessing steps, enabling an integrated approach to producing mRNA-based treatments.
The field of mRNA therapeutics is rapidly evolving, with mRNA technology offering promising new avenues for treating and preventing diseases. The production of mRNA, from design to large-scale manufacturing, relies on sophisticated equipment like the Applikon Mini bioreactor, which provides the necessary precision and scalability for this cutting-edge field. As biotech companies continue to explore and expand the applications of mRNA, the Applikon Mini bioreactor will remain an essential component in the development of the next generation of RNA therapies. Discover more about how the Applikon Mini bioreactor can support your mRNA production processes and pave the way for advancements in RNA therapeutics.
Feautured Products
SUPR single-use bioreactor
The SUPR single-use production bioreactor impresses with its simple, scalable and customer-friendly design. The cell culture reactor enables a seamless process transfer from R&D to production.

The SUPR bioreactor is available in the sizes 50L, 250L and 1000L.
AppliFlex ST GMP single-use bioreactor
The AppliFlex ST GMP is a configurable single-use bioreactor that meets all cGMP requirements for clinical production. The system simplifies Scale-Up, Scale-Down and Scale-Out. The fully closed system is customizable and reduces any risk of cross contamination.

The AppliFlex GMP is available in 500 mL and 3 L versions for both cell cultures and microbes.
Glass autoclavable bioreactors
The Applikon autoclavable bioreactor is a very popular bioreactor. It is available in 2, 3, 5, 7, 15 and 20-litre volumes. It is easy to adapt if your research changes. Thanks to the modularity and flexibility of the glass bioreactors, you can modify the system to fit any adaptations in process demands.
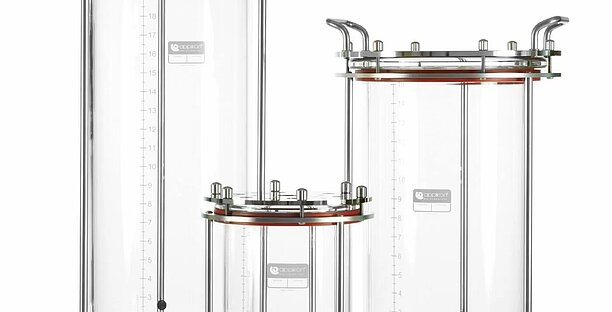
The glass bioreactors help support and optimise your research and process development. The systems are suitable for both cell culture and microbial culture applications.
V-Control
Developed in partnership with Emerson, V-Control is a process control platform designed as a 'one common-platform' solution for bioreactors from research to production.

V-Control offers a scalable software solution that overcomes the hurdles of scaling up.
Livit Flex bioprocess control system
The Livit Flex bioprocess controller is an intuitive and easily configurable bioprocess controller that fits any biotech upstream R&D application. Livit Flex can be configured as a single or dual control system for single-use or multi-use bioreactors to optimize bench space in the laboratory.

Livit Flex is ideal for use with single-use and reusable bioreactors up to 20L as well as our single-use pilot reactors.
Mini bioreactors MiniBio
The MiniBio is a true scale down of the traditional lab-scale bioreactor. The reactor is available in 250 mL, 500 mL and 1000 mL volumes and customizable to meet the demands of any bioprocess. It saves time, requires minimal bench space and generates more data with fully scalable results.

Despite its small footprint, the MiniBio can meet any process requirements. Whether for Batch, FedBatch or perfusion processes.
We are eager to receive your feedback
* Mandatory fields