Applications
Therapeutic proteins derived from mammalian cell culture
The demand for therapeutic proteins derived from mammalian cell culture is on an upward trajectory, fueled by the approval of newer products that often require administration in higher doses. Such products, including antibodies and receptor-binding proteins, necessitate the production of larger quantities than earlier therapeutic proteins. This shift underscores a continuous and pressing need to enhance the productivity of mammalian cell culture bioreactors, such as the Applikon Mini bioreactor , without the burden of significant additional investment in equipment. The Applikon Mini bioreactor stands out for its ability to provide a controlled and optimized environment for mammalian cell growth, especially for culturing CHO cells—a cell line that is extensively utilized in the biopharmaceutical industry.
The process of producing mammalian cell culture
The process of producing mammalian cell culture in a bioreactor involves several key stages, each crucial for optimizing cell growth and product yield. The Applikon Mini bioreactor is specially designed to cater to the intricate needs of mammalian cells, such as CHO (Chinese Hamster Ovary) cells, providing an ideal environment for their proliferation and production of biologics.
Initial preparation and sterilization
Before the culturing process begins, the bioreactor must be thoroughly sterilized to prevent contamination. Preparation of the Bioreactor
- Cleaning: The Applikon Mini bioreactor is thoroughly cleaned to remove any residues from previous cultures.
- Sterilization: Autoclaving or in-situ sterilization methods are employed to ensure all components in contact with the culture are sterile, eliminating the risk of microbial contamination.
The Applikon Mini bioreactor offers easy-to-use features for sterilization, ensuring a sterile environment for mammalian cell culture. This step is critical for maintaining the purity and integrity of the culture.
Inoculation and cell growth
The inoculation and cell growth phase is a critical juncture in the process of culturing CHO cells within the Applikon Mini bioreactor. This stage seamlessly integrates the meticulous preparation of the cell line with the advanced capabilities of the bioreactor to foster optimal cell development.
Cell line adaptation and expansion
Initially, CHO cells undergo a careful adaptation process to acclimate to the specific media and conditions provided by the Applikon Mini bioreactor. This adaptation is crucial for ensuring the cells' growth and viability are optimized for the bioreactor's environment. Following adaptation, the cells are expanded in flasks or smaller bioreactors, which serves to increase their quantity to the required inoculum density. This step is essential for preparing a robust starter culture that can thrive and proliferate once transferred to the bioreactor.
Inoculation into the bioreactor
Upon achieving the desired cell density, the next step involves the aseptic transfer of the CHO cell suspension into the sterile environment of the Applikon Mini bioreactor. This crucial phase marks the beginning of the cell culture process in the bioreactor, setting the stage for cell proliferation.
Optimization of culture conditions
The Applikon Mini bioreactor's advanced control systems come into play immediately after inoculation, regulating key environmental parameters such as temperature, pH, and dissolved oxygen levels. These factors are vital for cell growth and have a direct impact on the cells' health and productivity. The bioreactor's precise control systems, equipped with advanced sensors, allow for real-time monitoring and fine-tuning of these conditions. This ensures that the culture environment remains optimal at all times, facilitating sustained cell growth and viability.
In sum, the process from cell line adaptation and expansion through to the inoculation and subsequent growth in the Applikon Mini bioreactor exemplifies a well-orchestrated sequence of steps designed to maximize the efficiency and output of CHO cell culture. By meticulously preparing the cells and leveraging the bioreactor's sophisticated control mechanisms, it is possible to achieve high-density cultures and, consequently, higher yields of the desired bioproducts.
Nutrient Supply and Metabolite Removal
Mammalian cells require a continuous supply of nutrients and the removal of metabolic waste to maintain their growth and productivity. The Applikon Mini bioreactor is equipped with efficient feeding strategies and waste removal systems to support these needs. Its design facilitates the delivery of essential nutrients while simultaneously removing waste products, thereby preventing the accumulation of toxic metabolites that could hinder cell growth and product formation.
Harvesting and product recovery
The culmination of a successful mammalian cell culture process, particularly in the production of biopharmaceuticals using CHO cells, is the harvesting and product recovery phase. This crucial stage involves the systematic separation of the target bioproduct from the cell biomass and culture medium, a process optimized for efficiency and product integrity within the Applikon Mini bioreactor.
Harvest Timing
Harvesting is initiated when the cell culture achieves predetermined benchmarks of cell density and product concentration. These benchmarks are critical for ensuring that the product is harvested at its highest quality and yield. The timing is determined through rigorous monitoring of the culture parameters, including cell viability, density, and the concentration of the product of interest. This decision is crucial for maximizing product yield while maintaining high product quality.
Separation Process
The Applikon Mini bioreactor is engineered with features that support a gentle and efficient separation process, crucial for maintaining the integrity of both the cells and the bioproduct. The separation techniques employed can vary based on the nature of the product and the specifics of the culture system but typically include one or more of the following methods:
- Centrifugation: This process involves the use of centrifugal force to separate the cell biomass from the supernatant, where the product is usually found. Conditions are carefully optimized to ensure maximum recovery of the product while preserving its biological activity.
- Microfiltration or ultrafiltration: These membrane-based techniques are used to separate cells and larger particles from the product. They can be particularly useful for recovering products from the culture supernatant while minimizing product loss and maintaining high purity levels.
- Depth filtration: Often used as a preliminary step, depth filtration helps remove larger cell debris and aggregates, facilitating smoother downstream processing.
Product recovery and purification
Following initial separation, the recovered supernatant containing the product undergoes further purification steps to isolate the product with high purity and remove any impurities or contaminants. Techniques such as chromatography (affinity, ion exchange, size exclusion, etc.) and additional filtration methods are employed based on the specific characteristics of the product and the required purity standards. These steps are critical for ensuring that the final bioproduct is safe, effective, and suitable for its intended use.
Quality assurance
Throughout the harvesting and product recovery process, samples are continuously tested for quality parameters, including purity, potency, and the presence of contaminants. These quality control measures are essential for ensuring that the product meets all regulatory requirements and standards for biopharmaceuticals.
The Applikon Mini bioreactor, with its sophisticated design and control features, enables the efficient execution of these harvesting and product recovery processes. Its capabilities ensure that the transition from cell culture to product recovery is seamless and effective, leading to the production of high-quality biopharmaceuticals. This phase is not only pivotal in the bioprocessing workflow but also marks the transition towards the final stages of product development, where the focus shifts from cultivation to ensuring the therapeutic efficacy and safety of the bioproduct.
In summary, the Applikon Mini bioreactor is a versatile and powerful tool for mammalian cell culture, particularly suited for culturing CHO cells. Its design and functionality address the specific requirements of mammalian cell culture, from inoculation to harvesting, ensuring high-quality bioproducts. By leveraging the advanced capabilities of the Applikon Mini bioreactor, researchers and biotechnologists can push the boundaries of what's possible in the production of therapeutic proteins, vaccines, and other critical biologics. For those looking to excel in the field of mammalian cell culture, the Applikon Mini bioreactor represents a pivotal asset, embodying the pinnacle of bioreactor technology for mammalian cell culture.
Related documents
ABSTRACT
Single-use bioreactors have been increasingly used for animal cell culture in the biopharmaceutical industry. The interest for these systems lays in the considerable reduction of cross-contamination risk, the elimination of cleaning-in-place and sterilization-in-place, no need for cleaning validation, the decrease in production turnaround times and a reduction in validation time which shortens time to market.
In the present work, a Vero cell line used to produce viral vaccines was used by Intravacc to perform the cell and virus cultivations in Applikon’s newly developed small-scale customizable single-use bioreactors. The growth curves of Vero cells, were compared with the growth curves of Vero cells growing in conventional autoclavable glass bioreactors under the same conditions and in the same culture
volume. Subsequently, a virus for which vaccines are needed, EV71_C4, was grown on Vero cells. The single-use bioreactors are suitable for Vero cell culture and EV71_C4 virus propagation because there was no difference with respect to Vero cell culture and EV71_C4 virus culture between the glass bioreactor and disposable bioreactor. Thus the small-scale customizable single-use bioreactor holds promise for future production of viral vaccines.
ABSTRACT
With the increasing number of potential therapeutic molecules coming through from discovery, bioprocess scientists and engineers who have to guide process design and development have had to establish new technologies and methodologies for rapidly screening, defining, optimizing and characterizing process options. For these technologies and methodologies to be truly successful, they need to be scalable from operations at milliliter quantities in the lab to commercial scale operation. Scale down models (SDMs) need to provide assurance that product quality and process performance attributes are maintained between and within scales. Factors that contribute to this challenge include the needs for qualification, validation, regulatory requirements and the pressure to reduce cost and development time and resource requirements.
pdf | 4 MBABSTRACT
- NK-92 cells exhibit cytotoxicity against a variety of cancer cell lines, including leukemia’s, lymphoma’s, and malignant melanoma’s. This activity has lead to several small clinical trials as an anti-cancer immunotherapy, with larger multi-centre Phase II trials planned.
- The current production strategy relies on static culture techniques, resulting in large culture volumes and high cost. Volume and cost are further inflated by inefficient harvest and wash, with cell losses as high as 50%.
- Our GOAL is to enable cost-effective large-scale manufacture of clinical grade NK-92 cells.
Feautured Products
Glass autoclavable bioreactors
The Applikon autoclavable bioreactor is a very popular bioreactor. It is available in 2, 3, 5, 7, 15 and 20-litre volumes. It is easy to adapt if your research changes. Thanks to the modularity and flexibility of the glass bioreactors, you can modify the system to fit any adaptations in process demands.
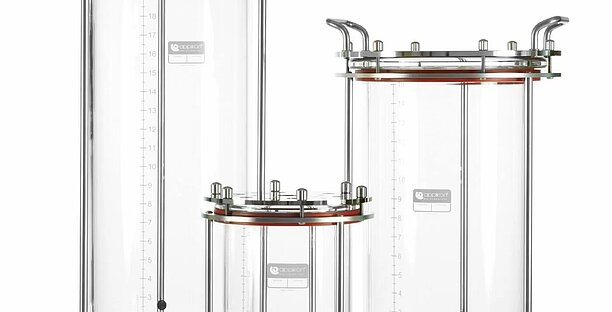
The glass bioreactors help support and optimise your research and process development. The systems are suitable for both cell culture and microbial culture applications.
Mini bioreactors MiniBio
The MiniBio is a true scale down of the traditional lab-scale bioreactor. The reactor is available in 250 mL, 500 mL and 1000 mL volumes and customizable to meet the demands of any bioprocess. It saves time, requires minimal bench space and generates more data with fully scalable results.

Despite its small footprint, the MiniBio can meet any process requirements. Whether for Batch, FedBatch or perfusion processes.
AppliFlex ST single use bioreactors
Thanks to 3D printing technology, each AppliFlex ST bioreactor can be uniquely configured to your individual process which includes custom impeller designs and various port connections.

The AppliFlex ST bioreactors come in 500 mL, 3 liter and 15 liter volumes. They are produced according to the high-quality Applikon standard and are interchangeable with Applikon’s glass bioreactors of the same volume.
Livit Flex bioprocess control system
The Livit Flex bioprocess controller is an intuitive and easily configurable bioprocess controller that fits any biotech upstream R&D application. Livit Flex can be configured as a single or dual control system for single-use or multi-use bioreactors to optimize bench space in the laboratory.

Livit Flex is ideal for use with single-use and reusable bioreactors up to 20L as well as our single-use pilot reactors.
SUPR single-use bioreactor
The SUPR single-use production bioreactor impresses with its simple, scalable and customer-friendly design. The cell culture reactor enables a seamless process transfer from R&D to production.

The SUPR bioreactor is available in the sizes 50L, 250L and 1000L.
Stainless steel bioreactors
Stainless steel bioreactors play a key role in the development and production biotech products. From bench to pilot to full production – the Applikon bioreactors simplify your scale-up through consistent design and scalable control solutions.

Our portfolio offers both standard systems for fermentation and cell cultures as well as customized systems.
my-Control bundle
The myControl Bundle consists of our small scale MiniBio bioreactors (250mL, 500mL and 1000mL), the myControl bioprocess controller, data acquisition software and the start-up kit. This space-optimized solution is the perfect partner for your research and development in the laboratory.

This versatile bioprocessing system can be used for both cell cultures as well as microbial cultures.
We are eager to receive your feedback
* Mandatory fields