BioSep perfusion device
The BioSep perfusion device represents a breakthrough in cell culture retention technology, offering unparalleled efficiency and reliability for high-density perfusion processes. Unlike traditional methods that rely on physical mesh or membrane systems, BioSep utilizes high-frequency resonant ultrasonic waves to separate cells, eliminating common issues and limitations associated with conventional devices.
Operating principle
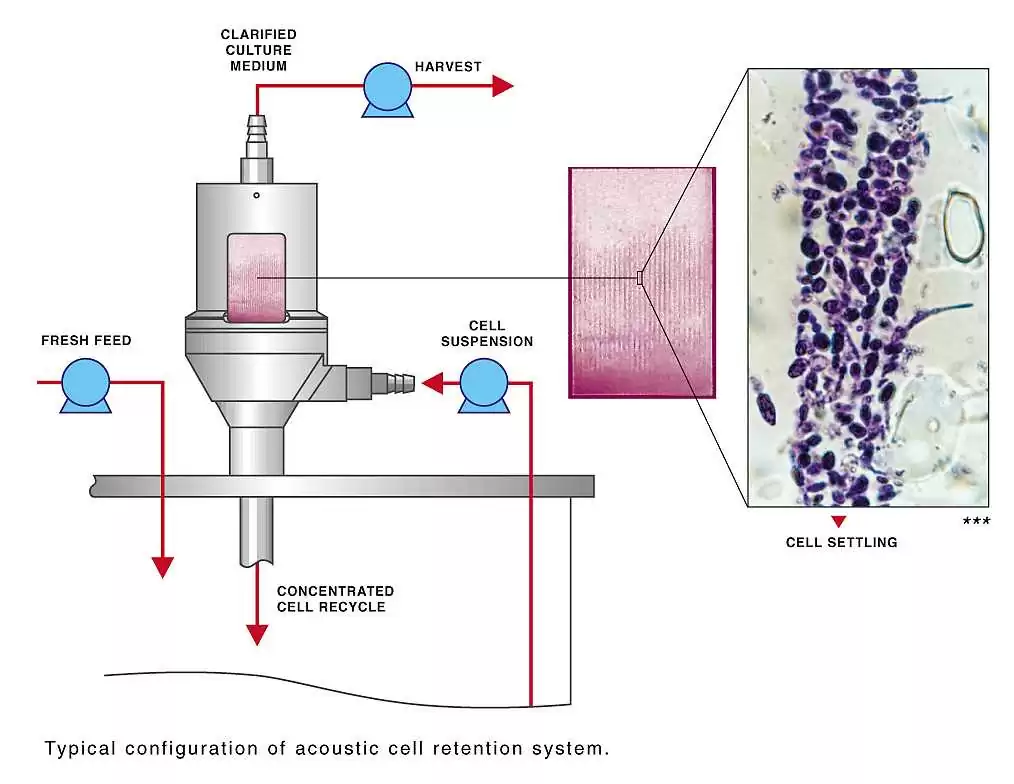
The Resonator Chamber of the BioSep device is seamlessly integrated into the bioreactor setup, with the cell suspension pumped into the chamber by a circulation pump. The flow is then divided between the harvest flow and the return flow, with the resonator controlling the flow rate. Ultrasonic forces within the resonator aggregate and hold suspended cells stationary against the flow, clarifying the harvest stream and promoting efficient cell retention. This continuous clarification ensures optimal cell viability and productivity throughout the perfusion culture. Planar aggregates formed by the ultrasonic forces are easily recycled back to the bioreactor for dispersion and continued cultivation, maximizing product yield and minimizing wastage.

Features
- Proven under cGMP conditions: The BioSep system has undergone rigorous testing and validation, ensuring compliance with current Good Manufacturing Practice standards.
- Easy to install and operate: The BioSep device's user-friendly design allows it to be seamlessly integrated into existing bioreactor setups, allowing for quick and hassle-free operation.
- Compatibility with any bioreactor brand: The BioSep system's versatility enables it to be used in conjunction with a wide range of bioreactor models, offering flexibility and convenience to users.
- Long-term cultivation capabilities: The BioSep device supports continuous cultivation for extended periods, enabling sustained production of high-quality cell culture perfusion systems for over six months.
- Non-fouling and non-clogging: The BioSep leverages acoustic resonance technology to ensure uninterrupted cell retention without the risk of fouling or blockage, maximizing productivity and efficiency.
- Cell-friendly operation: The gentle ultrasonic forces exerted by the BioSep device do not cause damage to the suspended cells, preserving their viability and functionality throughout the perfusion process.
- High separation efficiency: With a separation efficiency exceeding 95%, the BioSep device delivers consistent and reliable cell retention performance, enhancing overall process reliability and product quality.
Advantages of the BioSep perfusion system:
Continuous nutrient supply:
Perfusion bioreactors continuously feed fresh media containing essential nutrients, promoting sustained cell growth and productivity.
Enhanced cell density:
The continuous removal of waste products prevents the accumulation of inhibitory metabolites, allowing for higher cell densities and increased production yields.
Longer culture durations:
Perfusion cultures can be sustained for longer periods compared to batch cultures, leading to extended production runs and higher overall product yields.
Improved process control:
Perfusion systems offer greater control over culture parameters such as pH, dissolved oxygen levels, and nutrient concentrations, resulting in more stable and reproducible production processes.
Higher product quality:
The controlled environment of perfusion processes in biopharmaceutical production and perfusion bioreactors minimizes stress on cells and helps maintain product quality, resulting in higher purity and potency of the final biopharmaceutical product.


Facts and Figures
Applications:
- Cell cultures
- Perfusion cultures
Dedicated Bioreactors:
Category:
Applications:
Process development, Research Optimization, Pilot Plant, Manufacturing, Screening
Cultivation Volumes:
Types:
We are eager to receive your feedback
* Mandatory fields